22 Quantum Physics (A2)
22.1 Energy and Momentum of a Photon
-
Electromagnetic Radiation: Electromagnetic radiation has both wave-like and particle-like properties.
-
Photon: A photon is a quantum of electromagnetic energy that behaves as a particle.
-
Energy of a Photon: The energy of a photon is given by E = hf, where h is Planck's constant and f is the frequency of the radiation.
-
Electronvolt (eV): The electronvolt is a common unit of energy for particles, with 1 eV equivalent to 1.6 x 10^-19 J.
-
Photon Momentum: A photon has momentum, which is given by p = E/c, where c is the speed of light in a vacuum.
22.2 Photoelectric Effect
-
Emission of Photoelectrons: Photoelectrons can be emitted from a metal surface when it is illuminated by electromagnetic radiation.
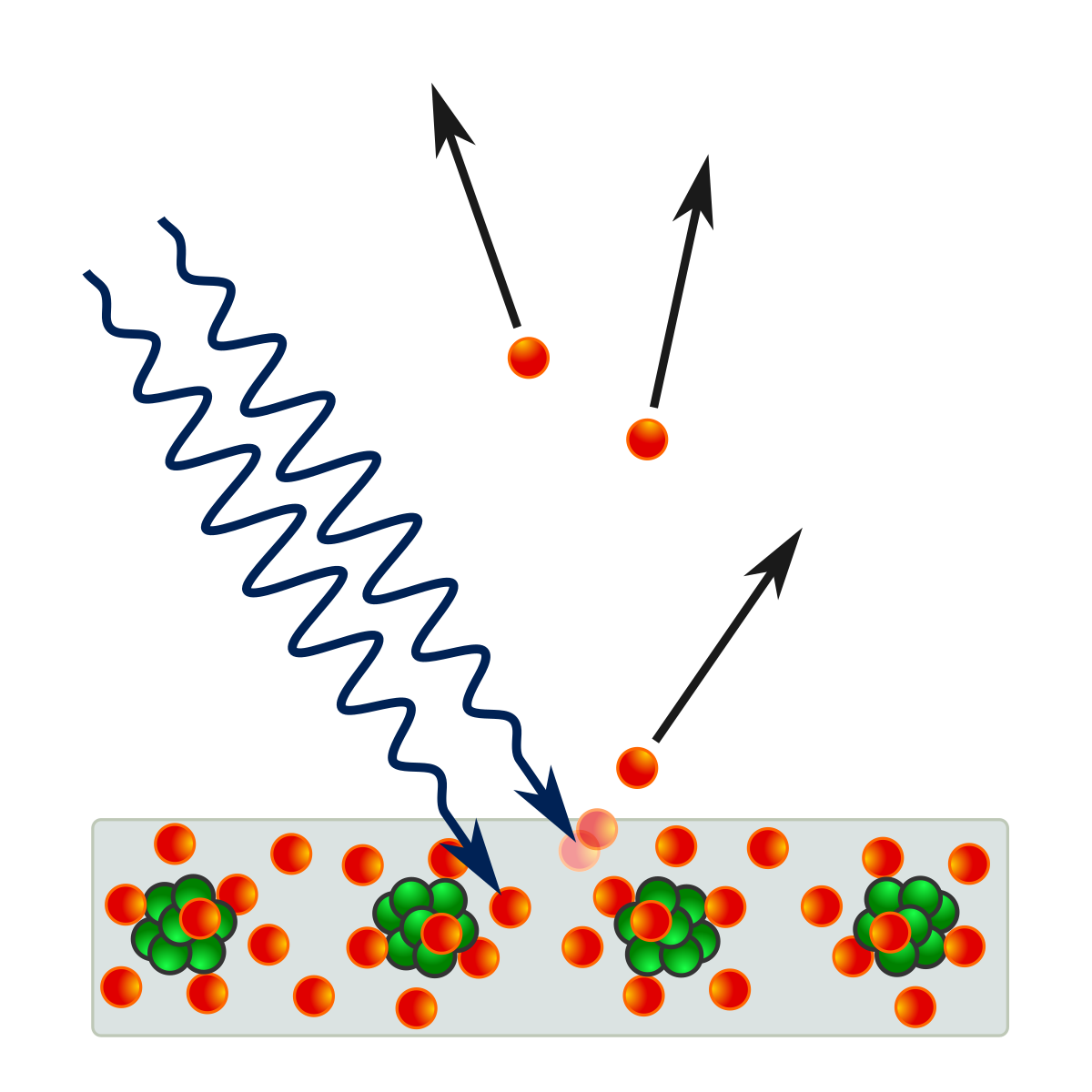 -- The Photoelectric effect
-- The Photoelectric effect -
Threshold Frequency/Wavelength: The minimum frequency or wavelength of radiation required to emit photoelectrons from a metal surface is called the threshold frequency or threshold wavelength, respectively.
-
Work Function: The energy required to remove an electron from a metal surface is called the work function, denoted by Φ.
-
Maximum Kinetic Energy: The maximum kinetic energy of photoelectrons emitted from a metal surface is given by the equation hf = Φ + 1/2mv2, where m is the mass of the electron and v is its velocity.
-
Intensity and Photoelectric Current: The maximum kinetic energy of photoelectrons is independent of the intensity of the incident radiation, but the number of photoelectrons emitted (i.e., the photoelectric current) is proportional to the intensity.
22.3 Wave-Particle Duality
-
Wave-Particle Behavior: The photoelectric effect suggests that electromagnetic radiation behaves as particles, while phenomena such as interference and diffraction suggest that it behaves as waves.
-
Electron Diffraction: Electron diffraction experiments demonstrate the wave-like nature of particles, where electrons exhibit diffraction patterns when passed through a diffraction grating.
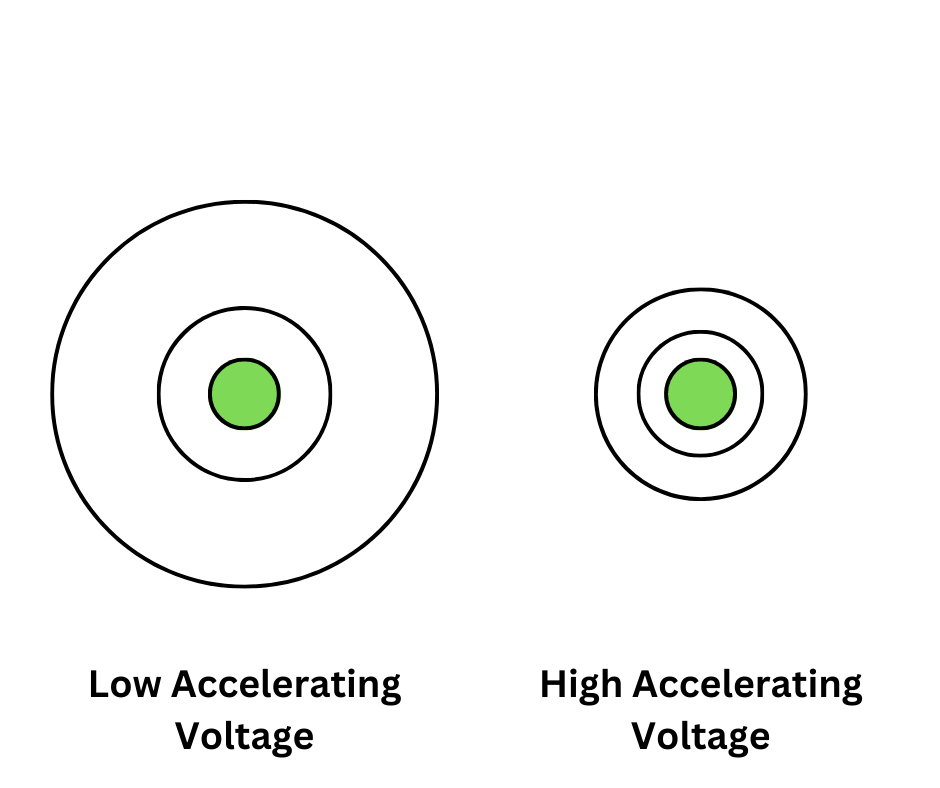 -- Differences in Accelerating Voltages in Electron DIffraction
-- Differences in Accelerating Voltages in Electron DIffraction -
de Broglie Wavelength: The de Broglie wavelength is the wavelength associated with a moving particle, given by λ = h/p, where p is the momentum of the particle.
-
Wave-Particle Duality: The wave-particle duality suggests that particles, including photons and electrons, exhibit both wave-like and particle-like behavior.
22.4 Energy Levels in Atoms and Line Spectra
-
Discrete Energy Levels: Electrons in atoms occupy discrete energy levels, and transitions between energy levels result in the absorption or emission of radiation.
-
Line Spectra: The appearance and formation of emission and absorption line spectra can be explained by transitions between energy levels in atoms.
-
Energy of Transitions: The energy of a photon emitted or absorbed during a transition between energy levels is given by hf = E1 - E2, where E1 and E2 are the initial and final energy levels of the electron.
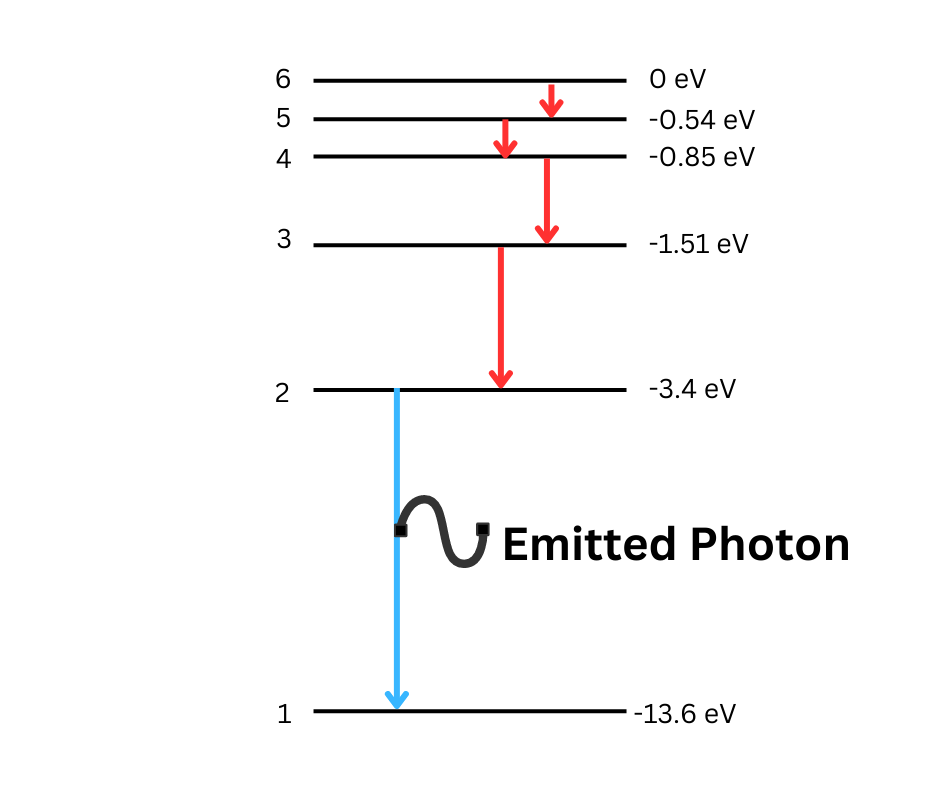 -- Transition energy through energy levels
-- Transition energy through energy levels